MI Express Urgent Care in Canton
44237 Michigan Ave, Canton, MI 48188
Monday - Friday : 9 AM - 7 PM
Saturday - Sunday : 10 AM - 6 PM
MI Express Urgent Care is located at 44237 Michigan Avenue, Canton, Michigan in a stand alone building at the intersection of Michigan Avenue and Sheldon Road.
MI Express Urgent Care is conveniently located at 44237 Michigan Avenue, Canton, MI 48188, just 2 miles west of I-275, at the intersection of Michigan Ave and Sheldon Rd, with easy access from Canton, Ann Arbor, Ypsilanti, and nearby cities. We are situated near popular stores, just 1 mile east of Kroger, Walmart, and Home Depot, and just 1-2 miles from Target on Michigan Avenue

At MI Express Urgent Care, we believe in providing timely and effective care when you need it most. Whether you’re dealing with a minor injury, illness, or a health concern that needs quick attention, our team is here to assist you. We are fully equipped with state-of-the-art technology and a team of experienced healthcare providers who are ready to treat a wide range of non-life-threatening conditions.
We understand that health issues don’t always happen during office hours, which is why we offer walk-in visits for your convenience. Additionally, we also offer online appointments to help you skip the wait and get the care you need right away.
Umayr Azimi, MD is an internist with medical expertise in acute care/hospital medicine, urgent care/occupational medicine, ambulatory/primary care medicine, and obesity medicine. He is double board-certified in Internal Medicine as well as Obesity Medicine by the American Board of Internal Medicine, and the American Board of Obesity Medicine.
Consult Your Primary Care DoctorNeed urgent care in Canton, MI? At MI Express Urgent Care, we offer walk-in services for patients of all ages, providing quick, reliable care in a friendly and welcoming environment. We’re here to treat your non-life-threatening health concerns when you need it most.

We provide immediate medical care and treatment for conditions like cold, flu, sore throat/fear of strep, UTI, pink eye, stomach problems, ear infection, rashes, insect bites, and other minor injuries.

We provide high-quality pediatric urgent care services that include treating common symptoms and illnesses ranging from influenza to bronchitis and viral infections and injuries, such as burns, fractures, sprains and strains, cuts, insect bites, sports injuries, etc.

With our flu shots, you can protect yourself from the seasonal flu. We offer flu shots for pregnant women, healthcare workers, people with existing health conditions, caregivers, and adults ages 65 or older.

We offer patients the option to pay for their medical services out-of-pocket, rather than using insurance. This will save you money and also help you avoid the hassle of navigating insurance claims and paperwork.

Whether you need a physical to ensure your readiness for a particular sport or a work physical to exhibit that you are healthy enough to work on a particular job, we can help.

We provide DOT physicals to commercial drivers across Canton, MI, to help them quickly obtain their commercial driving licenses. Our certified DOT examiners will conduct the exam adhering to all the guidelines set by FMCSA.
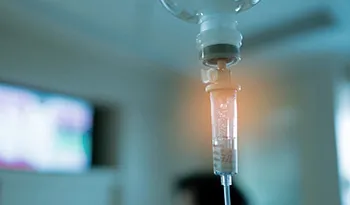
We offer a wide range of customized IV nutrition drips that include vitamins, minerals, and electrolytes to boost your energy and immunity, alleviate stress, and treat or prevent dehydration.

At MI Express Urgent Care, we are dedicated to delivering high-quality care in a welcoming and friendly environment. Our experienced healthcare team is here to assist you with any non-life-threatening health concerns. Whether you’re in need of a quick check-up or specialized treatment, you can trust us to provide prompt, professional care.
As we offer online appointments, you don't need to wait for hours to get seen.
We understand accidents and injuries can happen at any time, so we are open daily.
We are conveniently located in Canton, MI, so you can reach us easily and comfortably.
We offer onsite X-ray and lab services to ensure a quick and precise diagnosis that helps to begin your treatment on time.
Yes, many urgent care centers, including MI Express Urgent Care in Canton, MI, offer on-site X-ray services. Whether you have a minor fracture, chest discomfort, or need imaging after an injury, walk-in X-ray availability helps speed up diagnosis and treatment without the long wait.
To find the nearest urgent care in Canton, MI, simply search “urgent care near me” on Google or use map apps like Google Maps or Apple Maps. If you're in the Canton area, MI Express Urgent Care is conveniently located and well-rated for walk-in care.
No appointment is needed for most urgent care visits. MI Express Urgent Care welcomes walk-ins every day. However, if you prefer to skip the line, you can check in online for faster service.
Yes, many urgent care centers, including MI Express Urgent Care in Canton, are open on Sundays. Weekend hours make it easier to get care when your doctor’s office is closed.
Urgent care visits without insurance depend on the services needed. MI Express Urgent Care offers transparent pricing and self-pay options to make healthcare more accessible for everyone, even without coverage.